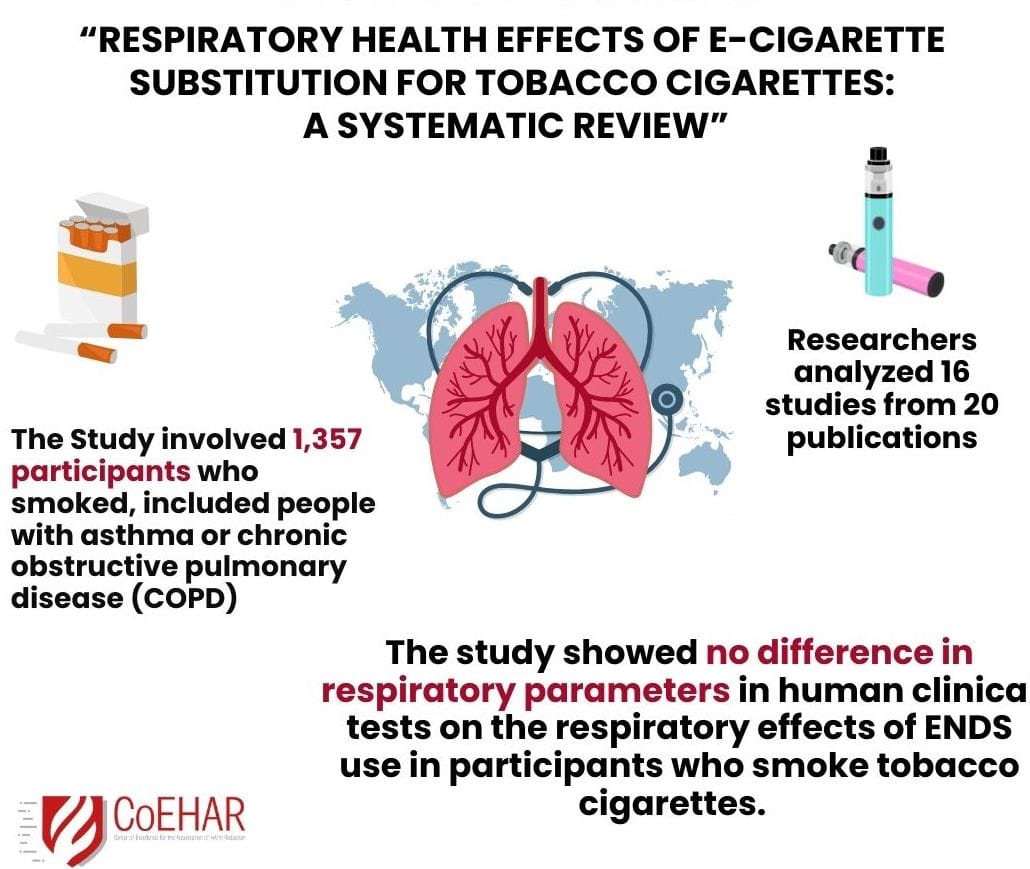
A new systematic review conducted on the available scientific research showed no difference in respiratory parameters in human clinical tests on the respiratory effects of ENDS use in participants who smoke tobacco cigarettes.
The substitution of electronic nicotine delivery systems for tobacco smoking can be a valid harm reduction strategy. For those seeking the final goal of complete nicotine cessation but who are not able to quit abruptly, using reduced risk products may improve their health while reducing the harm caused by smoking.
However, in order to give people who smoke relevant and reliable scientific data, is essential to evaluate the effects on health of e-cigarette substitution.
In their research “Respiratory health effects of e-cigarette substitution for tobacco cigarettes: a systematic review”, the researchers analyzed 16 studies from 20 publications. Their finding the large majorityof the studies showed no difference in respiratory parameters. This indicates that electronic nicotine delivery systems substitution for smoking likely does not result in additional harm to respiratory health.
The review included 5 studies from Greece, 4 from the United Kingdom, 3 from the USA, 2 from Italy and 1 each from Belgium and Hungary. The participants ranged in age from 18 to 73, comprising 1,357 participants who smoked. 6 studies included participants with asthma or chronic obstructive pulmonary disease (COPD). 9 studies presented follow-up data ranging from 5 days to 5 years: some findings showed improvements in lung function tests.
The respiratory health effects of ENDS substitution for smoking varied by health status and by the duration of ENDS use. For participants without respiratory disease, the acute studies did not show a clinically meaningful worsening of pulmonary function with ENDS use. Four acute and five short-term studies recorded no changes in healthy participants using ENDS.
“One of the problems found during our evaluation is that many studies were not of sufficient duration for observing any harmful or beneficial effects because these may take time to manifest. A reduction in symptoms may even take longer for patients with specific respiratory diseases. We urgently need long term studies and, above all, good quality research designs”.
In fact, the researchers observed a general low quality of the studies included in the review with ten of sixteen studies rated at high risk of bias.
Going beyond the quality assessments conducted in systematic reviews, the In Silico Team evaluated instances of reporting bias in the studies. Reporting bias occurs when the study authors maneuvers their discussion only to sources that conform to their desired conclusions. Another example is when authors unevenly highlighted one side of their study with the framing effect of language that focused on the loss of health or risks. In some studies, the authors stated that their findings showed an effect when the data were not statistically significant, meaning that there was no change in outcome with e-cig use.
In light of the findings of no change in respiratory function plus the presence of reporting spin bias, the In Silico researchers call for long term studies that include diverse participants and to assess smoking behavior and history. Furthermore, they note that exclusive ENDS use and dual use with cigarettes should be identified as separate categories for analysis and findings. Additional studies are still necessary to assess the potential benefits or risks of e-cigarette substitution for tobacco cigarette smoking.